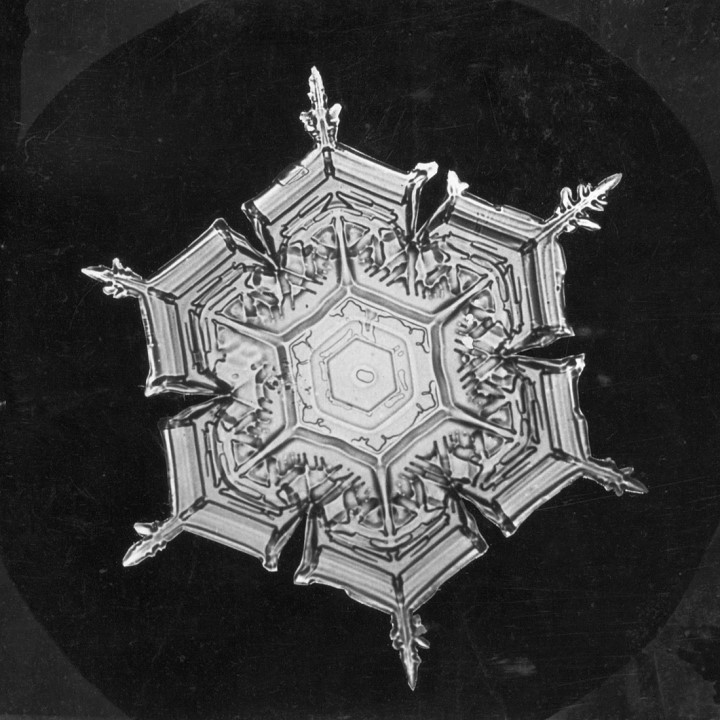
Mysteriously symmetric, beautifully complex, the how and why of snowflake formation.
So, why do they form in the first place? Ice does not form out of nowhere, the water must condense around something. This is the case for most phase changes, and these “nucleation sites” could take the form of small particles, from dust and pollen to smoke particulates. Remember those viral videos of turning a bottle of coke into a slushy with just a tap? It’s the exact same thing, the liquid is way below 0ºC but the lack of nucleation sites prevents freezing.
This is all well and good but it doesn’t explain how snowflakes form into their distinctive symmetric shape. It turns out, it’s all down to the hexagonal nature of H2O.
On the atomic scale, hexagons prevail when concerning water. Regular ice is composed of hexagonal sheets of water molecules stacked on one another, in a similar fashion to graphite, with hydrogen bonds between layers and molecules. However, upon closer inspection these sheets are not regular hexagons; they are chair-like and puckered, with a general hexagonal shape. This is caused by the internal bond angle of water (104.5º) not matching the internal angle of a flat hexagon (120º). However, this still doesn’t explain how the microscopic structure of water influences the macroscopic structure of the snowflake.

Figure 1 Microscopic to macroscopic hexagonal growth. The edges of each hexagon have two sites for hydrogen bonding, whereas the vertices have one. The edges are aligned on the green sides of the rectangle, with the vertices on the red sides.
For the structure to grow, water molecules collide at the edges of the hexagon and undergo hydrogen bonding. The likelihood of this is occurring is equal on all sides of the hexagon, which leads to water molecules clumping together to form regular, square sheets of hexagons. The two longer sides (marked in green on figure 1) of this newly formed rectangle have double the number of sites for hydrogen bonding than on the shorter red sides. This encourages growth on the longer sides (see green arrows). Addition of water molecules continues until all sides have equal probability of forming a hydrogen bond to another incoming water molecule, which is in the shape of a hexagon. This structure continues to grow larger, forming the basis of our snowflake.
Our final step, branching out. Typically, snowflakes have a hexagonal centre with branching arms outward. Why does this occur? Again, it is simply a matter of probability. As the hexagon grows larger, vertices become the most likely condensation site for water molecules, due to their relative isolation. This encourages elongation of these vertices into arms, which symmetrically branch out from the centre, forming our classic snowflake.
So, snowflake science boils down to nucleation, hydrogen bonding, and a small pinch of probability. Some might say, that’s almost kind of cool…
SOURCES
“Branch Growth and Sidebranching in Snow Crystals” Crystal Growth & Design, vol. 5, 2005, pp 1509–1525.
Snow Crystals vol 17, 2011. http://snowflakebentley.com/WBnews.htm#n17
How the crystal got its “six” March 15th, 2011. http://www.storyofsnow.com/blog1.php/how-the-crystal-got-its-six