iGEM is an international competition where teams of university students compete in designing a genetically engineered product to tackle a world problem. Oxford’s iGEM team for 2018 is made up of 10 undergraduate students who study a range of subjects including biochemistry, biology, chemistry, engineering and medicine. After a long process of designing and planning a project, the team is working in labs throughout the summer to develop their project before travelling to Boston, USA in October to present their work and compete in the international iGEM competition. Oxford’s team are engineering E Coli with the aim of creating a new therapeutic treatment for autoimmune diseases by altering the activity of a subset of T cells.
Role of T cells in the immune response
T cells are lymphocytes (white blood cells) involved in the adaptive immune response. T helper cells (Th) are a subset of T cells and are characterised by the presence of the CD4 protein on the cell surface. CD4+ cells interact selectively with antigen presenting cells that express specific antigens – molecules capable of initiating an immune response – on their cell surface. The interactions of Th cells upon activation by a complementary antigen presenting cell will result in the release of small proteins – cytokines – that influence the activity of other immune cell types. Specifically, activated Th cells can enable the maturation of B cells to upregulate the production of antibodies, as well as the proliferation of cytotoxic T cells (cells which kill infected body cells). Our project focuses on enabling the detection of a particular subset of T helper cells – Th17 cells. Th17 cells produce IL-17 – a proinflammatory cytokine – and the incorrect balance and functioning of these cells is associated with the development of multiple autoimmune diseases, such as Crohn’s disease or lupus.
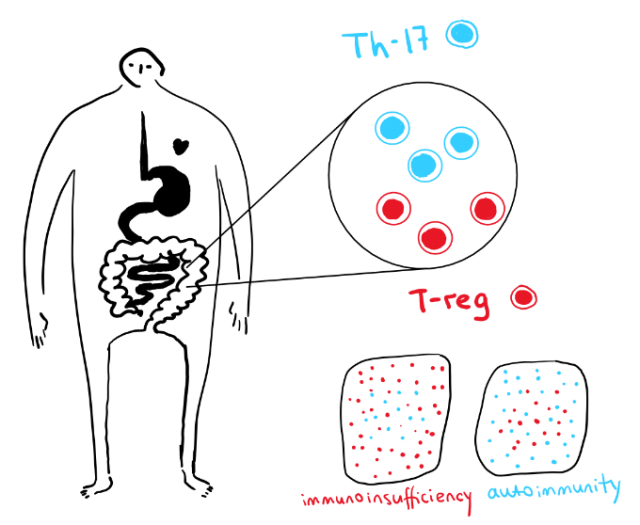
Treg cells, another subset of T cells, can prevent the immune system from becoming overactive. They do this by suppressing the action of T helper and cytotoxic T cells, thereby preventing the development of an excessive inflammatory state and can limit self-reactivity, thus limiting the development of autoimmune diseases. Figure 1 demonstrates the fine balance that must exist between these two types of T cell to avoid the development of immunodeficient and autoimmune states.
Autoimmune diseases
Autoimmune diseases encompass a range of 100 known diseases that have a huge clinical burden and are characterised by the following features:
- Autoimmune diseases result when the body’s immune system incorrectly targets and attacks the body’s own tissues.
- The diseases have a broad range of symptoms and complications, with examples including Crohn’s disease, type 1 diabetes and multiple sclerosis. Autoimmune diseases have a long-term effect on the health and quality of life of patients, as well as having a huge cost and burden on healthcare services.
- The prevalence of autoimmune diseases is rising in both the developed and developing world, and there are significant global inequalities in the treatment outcomes.
- Current treatments for autoimmune diseases, such as corticosteroids, are associated with a range of negative side effects and often need to be taken on a regular basis to manage the disease symptoms.
- Common treatments focus on symptom management and will frequently result in only sub-optimal control, as well as being less accessible in the developing world.
Autoimmune diseases can result from an imbalance in the populations of the subtypes of immune cells within the body, such as the balance of Treg and T helper cells. We have placed particular attention on autoimmune diseases in developing countries where bacteria, such as segmented filamentous bacteria (SFB) are highly prevalent in water sources or in areas close to livestock. SFB are bacteria that tightly adhere to the lining of the gut lining and can modulate host immune responses. For example, SFB has been shown to promote the development of lymphocytes and the differentiation of Th17 populations, resulting in the production of IL-17a. As a proinflammatory cytokine, an elevated level of IL-17a due to SFB infection is associated with the development of autoimmune diseases, such as autoimmune epilepsy and multiple sclerosis.
The aim of our project is to rectify the notable yet unmet global need for better treatments for autoimmune diseases by creating a probiotic that will alter the immune system’s activity to prevent the self-destruction of tissues.
The microbiome and probiotics
The microbiome refers to the population of symbiotic microorganisms that normally reside within the human gut and play critical roles in maintaining human health. It is thought that there are around 100 different species of microorganism in the gut in infants, and this number steadily increases to over 1,000 different species in adults. The composition of the microbiome is highly variable between individuals, and is determined by multiple environmental factors such as diet, exercise levels, exposure to antibiotics and age. Changes in microbiome composition, most notably a reduced diversity of the component species, is associated with an increased likelihood of developing inflammatory bowel diseases, autoimmune diseases and obesity.
The aim of our iGEM project is to design a probiotic – a live bacterial product that will result in beneficial effects in the human body – that can be used to treat autoimmune diseases. The idea is that the genetically engineered bacteria will be digested orally – either in a pill or yoghurt formulation – and the bacteria will reside within the normal microbiome. Using probiotics to target specific diseases is a relatively new phenomenon, but specific probiotic-based treatments include treatments for infectious diarrhoea and C. difficile infections.
Our project
Our project aim is to engineer E coli so that, when digested as a probiotic, is can reside within the microbiome and regulate the relative populations of Treg and T helper cell populations within the body. Our engineered E coli will contain a system that detects the increased concentration of Th-17 cells and responds by secreting an appropriate amount of IL-10 (an anti-inflammatory cytokine) to prevent overactivity of the immune system and excessive inflammation and self-destruction. A negative feedback loop will indirectly sense the concentration of Treg cells and, when high, will prevent the release of IL-10 to prevent the excessive suppression of the immune response and the resulting immunodeficiency.

We are engineering E coli to detect nitric oxide, which is released continuously by Th cells. As NO is a small molecule that is permeable through the outer cell membrane of E coli, NO can be used a suitable biomarker for the size of the population of T helper cells. An excessively high concentration of NO is indicative of a high activity of Th cells, and therefore signifies an overactive immune system.
Our engineered bacteria will use the SoxR/SoxS promoter system to detect NO and induce the transcription of IL-10, as demonstrated in Figure 2. This is a system in which NO will bind to the SoxR protein to activate it, and then the active NO-SoxR complex will bind to the SoxS promoter region. The transcription of IL-10 will be under the control of the SoxS promoter, meaning that the activation of the SoxS promoter due to the binding of the active NO-SoxR complex will result in the transcription of IL-10. The transcribed IL-10 should be secreted into the surrounding environment via a secretion system. IL-10 is an anti-inflammatory cytokine, meaning that its release should suppress the inflammatory, autoimmune state in the surrounding environment in the body.
Our engineered bacteria will also contain an adenine-induced negative feedback loop to inhibit the overexpression of IL-10 and the resulting immunosuppression. Adenosine is a small molecule that is synthesised by a series of reactions involving the metabolism of ATP by Treg cell surface enzymes, meaning that adenosine is a suitable biomarker of Treg population size. Our engineered bacteria will express an outer membrane-anchored hydrolase enzyme that will convert adenosine into adenine, which is a signal that our E coli system can detect. In this manner, adenine can act as an effective biomarker for Treg activity, thus representing a situation in which there is sufficient control on the immune response (and therefore a situation where releasing IL-10 is no longer appropriate).
Our feedback loop consists of an adenine riboswitch that controls the transcription of sRNA that will bind to IL-10 mRNA and acts to inhibit the translation and release of IL-10 (see Figure 2). The absence of adenine will prevent the activation of the riboswitch, therefore inhibiting the production of the sRNA to enable the undeterred translation of IL-10. However, when adenine is present at specific concentrations, the riboswitch will be activated; sRNA will be transcribed and will act to inhibit the translation of IL-10. In this manner, excessive production of IL-10 will be prevented and this will enable an optimal, personalised dose of IL-10 to be delivered to the patient.
We are proposing that the cultures of probiotic bacteria would be digested using a live yoghurt or tablet, since this would avoid the need for injections, training for appropriate use and the associated risk of infections. The personalised dose of IL-10, along with the adenine-induced negative feedback loop, means that the treatment should not be associated with the side effects of common treatments for autoimmune diseases, such as immunodeficiency. We have had many discussions with researchers and clinicians working in Oxford and beyond, and believe that our design has the potential to be a successful treatment that could overcome the common problems associated with current treatment options for autoimmune diseases.
Our progress
Our aim for the summer is to engineer the E coli and to run in vitro proof-of-principle experiments to demonstrate that our design works in an in vitro set up. We’re also gaining feedback from clinicians, researchers and patients to understand how we can improve our design to maximise the effectiveness and benefits for patients. Aside from the main product, we’ve also held sessions at various summer schools in Oxford to teach school students about synthetic biology, and are planning various outreach events across Oxford. Cureently, preparations are underway for the presentation of our work in Boston at the international iGEM Jamboree, which should be the highlight of the iGEM experience. We’d love for you to keep track of our progress or ask any questions by following us on Twitter (@OxfordiGEM) or Facebook (Oxford iGEM).
Written by Ellie Beard
Drawings by Jhanna Kryukova





