In the 1982 classic Bladerunner, synthetic beings on the boundary between life and artifice known as replicants serve as humanity’s slaves. Made from the same organic material as us, they are expertly bioengineered to be improved versions of the humans they are based on. Masterful manipulation of life in this way could be a reality sooner than we might think.
Scientists in the US have made the first living machines by piecing together cells from frogs into designs which function like living robots (Kriegman et al., Proceedings of the National Academy of Sciences 2020). Their creations can pick up and carry objects, move around by themselves and even heal after being damaged. However, unlike replicants, these machines are described as being as different as possible from existing species, whilst still being made of natural cells.
Traditional robots, the researchers say, degrade over time, presenting hazards to health and the environment. However, when one of these ‘living’ robots dies, it simply transforms into dead tissue, which is biodegradable by definition. You’ll find no whizzing gears or flashing steel here, instead these robots resemble little more than strange fleshy blobs. About 1mm in size, they eerily scoot around. In addition, living matter has some significant advantages, including the ability to self-repair, and compatibility with natural life.
Design by directed evolution
In order to design the robots, Kriegman and colleagues at the University of Vermont created an algorithm. The algorithm generates random designs, then tests each of these in a simulated environment. Designs that have the desired properties, such as the ability to move quickly, are retained whilst deficient designs are killed, or deleted. The process is then repeated, generating diverse and novel machines, in a mechanism that mimics the natural process of evolution. For example, a tendency for these robots to herd cellular debris together was observed. This was then introduced as a constraint to the evolutionary algorithm and designs were produced which were much better at clearing debris in this way.
Blackiston and others at the Allen Discovery Centre, Tufts University, then manually built these machines. They took stem cells from frogs, then differentiated them and pieced them together by simply using forceps. Watching their behaviour, they sent the designs that worked back to the computer scientists, who used them to improve their algorithm. Two types of cells were used: skin cells, which are passive, and heart cells, which can contract. Heart cells function as a sort of engine, projecting the robot around, whilst the skin cells provide surface area to interact with the environment.
The researchers who developed this strange invention have called the robots Xenobots, the name taken from Xenopus laevis, the frog species whose cells the Xenobots are made from. The ancient Greek root Xenos means ‘the stranger’, or ‘the alien’, a suitable name for this unique creation. Xenobots are something very new: both a living entity and a programmable machine. Unlike other new lifeforms, which have been made using synthetic DNA inserted into empty cells, Xenobots are repurposed natural life, like redeployed soldiers.
Surprisingly lifelike
The most surprising aspect about the Xenobots is the startling ways they behave. They shoot back and forth, or spin around. Xenobots designed with a hole in the middle have even been seen to pick up objects. As the developers wrote, the Xenobots display remarkable self-organising abilities without having any kind of nervous system. As an example, without any input from the scientists the heart muscle cells coordinated their contractions, which then aided the robot’s movements.
Such properties were unintended consequences. Although certain behaviours can be selected for in the evolutionary algorithm, ultimately little is understood. It is unclear, for example, why one individual robot travels faster than another. In other words: we can produce a robot that goes fast, but we cannot predict what any particular arrangement of cells will do. The individual cells cooperate to form complex behaviours, organically communicating that a certain geometry of cells leads to one behaviour, another to a completely different one. The cells are genomically frog cells but do not appear to resemble frog anatomy or behaviour. The team hopes that their work can lead to better understanding of this murky chemical intercellular organisation, but this gap highlights how little is currently known.
Tiny agents in the blood stream
The possible applications of this technology are diverse and powerful. Imagine a team of microscopic Xenobots hoovering up plaque in an artery or attaching themselves to cancer cells. Xenobot teams could be deployed to drop off a drug in a specific location, or even carry out tiny surgeries. The robots could be constructed from a patient’s own cells to prevent the immune system from destroying them, and the lack of a metabolism means they cannot linger long enough to cause substantial damage.
Significant challenges remain before Xenobots can, say, be deployed to clean up radioactive waste in the oceans. Manufacturing each design in a painstaking, manual process is highly inefficient. Whilst the work so far shows proof of concept, a more scalable approach to manufacture is needed. Before serious technological application is possible, serious understanding of behaviour is also needed, and safety will have to be proved.
But these hills are not insurmountable, and our high-tech future could be a lot squishier than perhaps imagined.
—————————-
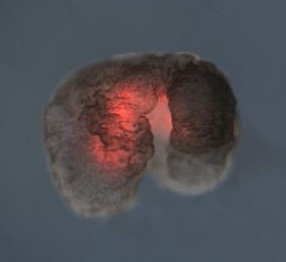
Title image:
A Xenobot with red fluorescent dye marking cardiac contractile cells. Image from “A scalable pipeline for designing reconfigurable organisms” by Kriegman et al., licensed under CC BY 4.0
Further information:
https://www.pnas.org/content/117/4/1853
https://www.youtube.com/watch?v=aQRBCCjaYGE